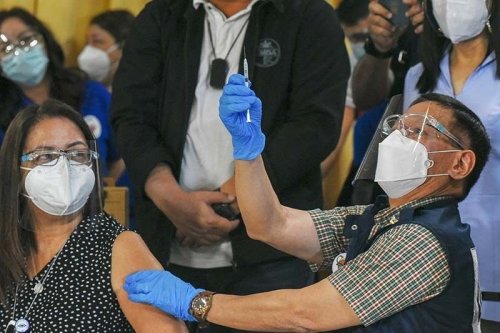
FDA needs more data on use of Sinovac COVID-19 jab for kids
FDA needs more data on use of Sinovac COVID-19 jab for kidsA Sinovac vaccine against the Covid-19 coronavirus is seen at a psychiatric hospital in Banda Aceh on February 6, 2021. MANILA, Philippines — Chinese drugmaker Sinovac Biotech needs to submit updated data for the emergency use application of its COVID-19 vaccine for children aged three to 17, the head of the country’s Food and Drug Administration said Monday. “We have asked for more clinical trial data because the sample size they submitted was too small,” FDA Director General Eric Domingo said in Filipino. “Once they submit the updated data, their application will be reviewed again by our experts to determine if it can be allowed,” he added. Domingo also said Pfizer submitted an application for the emergency use of its vaccine for children between five and 11 years old.
Source: Philippine Star December 13, 2021 16:14 UTC